|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
| cyclopropane | cyclobutane | |
| Cyclobutane's lowest-energy conformer is slightly puckered -- angle strain is increased in order to relieve torsional strain! | ||
Planar cyclopentane has almost-normal bond angles of 108°. Nevertheless, to avoid torsional strain the molecule bends into the so-called envelope conformation, in which one of the carbon atoms is bent out-of-plane. Notice that this staggers the hydrogens on that atom relative to those on adjacent carbon atoms.
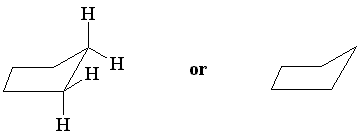 |
||
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.