|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
Look at your model of ethane. Notice that the central carbon-carbon bond rotates freely. This means that the hydrogens on the adjacent carbon atoms can be either alternating or all lined up; the appropriate terms are staggered and eclipsed. Since hydrogen atoms have size and so interfere with each other, the staggered conformation of ethane is lower in energy than the eclipsed conformation. The eclipsed conformation has what we call torsional strain because of the interactions of eclipsed hydrogen atoms.
The way we emphasize conformation about a particular bond is with a Newman projection. To understand a Newman projection, imagine that you are sighting down the carbon-carbon bond in ethane (take your model and do so now!) The carbon atom behind is represented by a large circle; the hydrogens attached to each carbon can be clearly seen in the projection.
| staggered ethane | ||
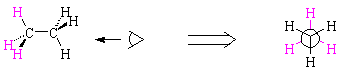 |
||
| eclipsed ethane | ||
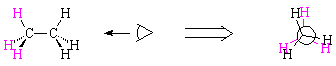 |
||
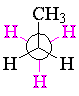
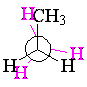
In the same way, propane can be either staggered or eclipsed about either of the carbon-carbon bonds.
| staggered propane | eclipsed propane | ||||
 |
 |
||||
However, butane has another bit of conformational information: when you look down the central carbon-carbon bond, the staggered form can have the methyl groups either adjacent (gauche) or opposite (anti). The anti conformation is lower in energy than the gauche. While this makes little difference in most of the chemistry of butane, conformational considerations become important when we consider cycloalkanes.
| anti butane | gauche butane | ||||
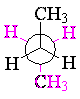 |
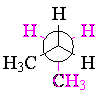 |
||||
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.