|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
Boat cyclohexane has no angle strain, but there are several eclipsed interactions between neighboring hydrogens. The actual "boat" conformation is the so-called twist boat, in which the eclipsed interactions are relieved by twisting around some of the carbon-carbon bonds. Nevertheless, the boat conformation is still relatively high in energy because of the unavoidable flagpole interactions between the hydrogens on the insides of the "prow" and "stern." The Newman projection below emphasizes the eclipsed interactions along the "gunwales" of the boat.
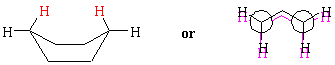 |
The flagpole hydrogens are highlighted in red. | |
| Boat cyclohexane | Twist Boat cyclohexane | |
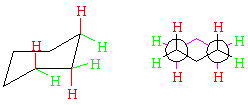
Chair cyclohexane has neither angle strain nor eclipsed interactions! In fact, it has zero strain energy. If you build a model of chair cyclohexane, you will notice that there are two types of hydrogens, depending on whether they point up or down with respect to the ring, or point along the ring "plane." These types are called, respectively, axial and equatorial, and are color-coded below. The Newman projection emphasizes that all hydrogens are staggered; if you examine your model you will see that this is true all the way around the ring.
 |
|
| When you "ring-flip" from one chair form to the other, axial and equatorial hydrogens change places. | 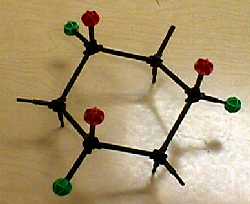 |
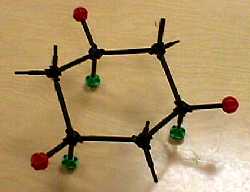 |
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.