J.W. Linnett, Nature, 1947, 160, 162-3
copyright © 1947 Macmillan Magazines Ltd.
Structure of Ethylene Oxide and Cyclopropane
A. D. WALSH1 has suggested that the properties of cyclopropane and ethylene oxide are better accounted for by the formulæ I (three equivalent resonance forms) and II than by III and IV:
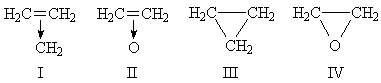
One consequence of this suggestion is that, in the CH-bonds, the carbon orbitals should be of the sp2 hybrid type, rather than the sp3 hybrid suggested by III and IV. In 1945 I obtained the force constants of a number of CH-bonds and found the following: methane, 4.97 (in the usual units); ethane, 4.81; ethylene (sp2 bonds), 5.12. Fox and Martin3 have suggested that in paraffins the CH-force constant is: CH4, 5.04; CH3 group, 4.75; CH2 group, 4.56; CH group, 4.56. The force constant of the CH-link in cyclopropane and ethylene oxide might, therefore, prove valuable in deciding between the above structures.
From the Raman4 and infra-red5 spectra of cyclopropane it has been decided that the CH-valency vibration frequencies are: A1', 3,000; E', 3,000; A2", 3,050; E", 3,080 cm.-1. The derived force constants are: 5.04, 5.04, 4.91 and 5.02. The mean is 5.0. (The same values of the fundamental constants have been used as in my previous paper2.) The Raman4 and infra-red5 spectra of ethylene oxide indicate that the CH-valency vibration frequencies are A1, 3,000; B1, 3,000; B2, 3,062; A2, 3,062 cm.-1. The derived force constants are 5.04, 5.04, 4.95 and 4.95. The mean is again 5.0. Ethylene sulphide is also of interest. Thompson and Dupré, having examined the Raman and infra-red spectra, suggested for the CH-valency vibrations: A1, 2990; B1, 2,990; A2, 3,080; and B2, 3,080 cm.-1 6. The derived force constants are 5.00, 5.00, 5.02 and 5.02. The mean may be taken as 5.0. In all these three compounds the CH-force constant is 5.0.
For the CH-bond in a methylene group in an ordinary paraffin chain, we would expect the force constant to be about 4.6 (Fox and Martin), and certainly not greater than 4.8 (CH3-group). The force constant in the methylene groups in these ring compounds is certainly significantly greater than this. It is, moreover, nearer the ethylene figure of 5.1 than to the figure for a methylene group in a paraffin. (It seems proper to make the comparison with the CH2-group in a paraffin chain, though it may be noted that the CH-force constant in these ring compounds is not appreciably different from that in methane itself.) The conclusion to be made from these calculations must be that the force constants indicate that the CH-links in these ring compounds approach those in ethylene. So the calculations provide a measure of support for Walsh's suggestion. Of course, the CH-force constant might change simply as a result of the distortion in the angle between the bonds which form the ring. However, such a change is hypothetical, and it is, therefore, rather unsatisfactory as an explanation of these results.
The possible usefulness of the above calculations became apparent to me after hearing a lecture by Dr. Walsh on this and related topics.
J.W. LINNETT
Inorganic Chemistry Laboratory
Oxford
May 17.