|
|
Organic ChemistryUsing Molecular Models |
| Return to previous page | Return to Index | Go to next section |
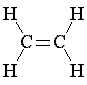
Build a model of ethene ("ethylene"), and compare it to the models shown below. The "bonds" are arranged at normal trigonal planar angles of 120°. Notice that the online model does not show the double bond!
 |
 |
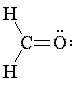
Carbon-oxygen double bonds - carbonyl groups - may be represented using the gray and red half-bonds. To represent a carbonyl group, first snap together one red and one gray half-bond; then snap a gray end-piece into the gray end of the double bond.
 |
 |
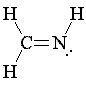
Carbon-nitrogen double bonds are rarer in organic chemistry, and the fact that the nitrogen's valence is not filled means that a group may be bonded to nitrogen. To represent a carbon-nitrogen double bond, take a gray double-bond piece. Snap a gray end-piece into one end to represent carbon, and a blue tetrahedral piece into the other end for nitrogen.
 |
 |
Triple bonds are represented using the gray triple-bond pieces. Each piece represents two carbon atoms with a triple bond between them and one open valence for each atom. Notice that triple bonds have a normal bond angle of 180°. Carbon-nitrogen triple bonds are also possible (such bonds are referred to as cyano or nitrile groups), but there is no distinct way to represent them with a "sticks-only" kit. However, Darling Models has recently added Atom Visions pieces to its kits which allow one to represent ball-and-stick models; using these pieces, one can distinguish unambiguously between e.g. propyne and acetonitrile.
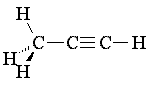 |
 |
 |
|
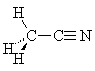 |
 |
 |
| Return to previous page | Return to Index | Go to next section |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.