|
|
Organic ChemistryUsing Molecular Models |
| Return to previous page | Return to Index | Go to next page |
| methane | ammonia | water |
To represent methane (CH4), you may use four white balls (representing hydrogen atoms) attached to the black tetrahedral center; ammonia (NH3) and water (H2O) can be represented by using three and two white balls, respectively. However, larger molecules will have too many hydrogens for this method to be practical given your limited supply of white balls. One alternative is to represent a lone pair of electrons by a colored ball, and let blank ends represent hydrogen atoms! Also, divalent oxygen (as in water) can be represented by a single red tetrahedral piece.
| Molecular Visions models of methane | ||
 |
 |
|
| Molecular Visions models of ammonia | ||
 |
 |
 |
| (red balls used to represent lone pairs of electrons) | ||
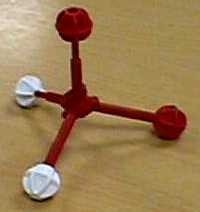
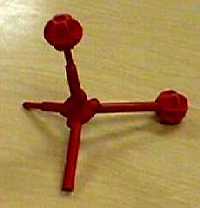
| Molecular Visions models of water | ||
 |
 |
 |
| (red balls used to represent lone pairs of electrons) | ||
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.