But is it Art? Representing structure in chemistry
previous Molecules of the Month
This article adapted from D. Berger, National Forum, Spring 2001.Anyone who has paged through a book on chemistry has seen some representation of a chemical compound. The compound may be simple or complex, but the representation has to satisfy certain requirements: the trained eye must be able to discern both structure and function, yet the picture must be quickly reproducible by an artistic tyro. Chemistry is a profoundly visual discipline. To do chemistry on anything more than an elementary level, you have to deal with objects in three dimensions, usually via representations in two. Chemists often think in such representations, and creative chemists use them to build mental structures as elegant and rich as a Tolstoy novel.
References: R. Hoffmann and P. Laszlo, "Representation in Chemistry," Angewandte Chemie International Edition in English, 30, 1-16 (1991); chapters 14-16 of Hoffmann's The Same and Not the Same (Columbia University Press, 1995); and Weininger, "Contemplating the Finger: Visuality and the Semiotics of Chemistry," HYLE, 4, 3-27 (1998).
Chemical representation is not abstract art; it is intended to represent something visual. It has as much kinship with the diagrams in your VCR manual as it does with a 17th-Century pastorale or the 11th-Century Bayeaux "tapestry." But what is it that is represented? And how is it done? Let's use a simple organic compound, C6H12O, as an example. For some purposes, this representation, the molecular formula, is a perfectly adequate description. It gives the type and number of atoms in the compound, and distinguishes it from other organic compounds such as C6H6 or C4H11N.
For most other purposes "C6H12O" is useless. By my count there are 472 chemically reasonable ways of arranging six carbons, twelve hydrogens and one oxygen. How can we unambiguously represent one of the possibilities?
One simple way, and the first way taught to students, is to draw a map of the molecule, showing each atom's attachments to its neighbors (Figure 1a or 1b). The map shows how a particular compound, 2-methylcyclopentanol, is put together and distinguishes it from other isomers of C6H12O. But organic chemistry is densely populated with carbons attached to hydrogens, and this sort of thing quickly becomes unwieldy.
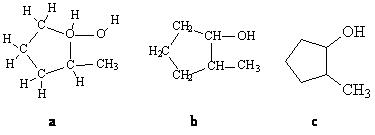
Figure 1. Structural representations of 2-methylcyclopentanol.
What's more, Figure 1a assigns apparently equal importance to every atom; chemically we don't want that. Carbon-hydrogen units are chemically inert, and we want to call attention to the points at which chemistry is most likely to happen–just as ancient Egyptian or medieval European artists made the most important people in a painting larger than surrounding objects–without losing sight of the carbon and hydrogen scaffolding on which the molecule is built. And why write a symbol for every carbon and hydrogen atom? What is normally done is to leave out carbon and hydrogen atoms entirely! Only the bonds between carbon atoms are shown; a carbon is assumed at each vertex. This shorthand takes advantage of the fact that, almost without exception, carbon forms four bonds: no more, no less. "Missing" bonds are assumed to be to hydrogen atoms; compare Figure 1a with Figure 1c. "Heteroatoms"–atoms other than carbon and hydrogen–are shown explicitly, as are the hydrogens attached to heteroatoms. This is appropriate; it is at or near heteroatoms and their attendant hydrogens that organic chemistry largely takes place. Such schematic drawings greatly simplify the task of chemists, who may have to draw complex structures at a moment's notice, in an informal discussion or during a formal talk.
Notice that, in Figure 1c, a carbon is shown explicitly: CH3. This is perfectly acceptable so long as all attached hydrogens are also shown.But there are other structural features to be represented. All molecules with more than three atoms have the possibility of three dimensions: the geometry of carbon, with its four bonds, was proved to be tetrahedral more than a century ago. Organic molecules can be and often are represented by flat structures, but they exist in space. We could draw a fully proportional model, with highlights and shadows and the other appurtenances of artistic perspective, but that would make chemical structures inaccessible for any but trained artists. Instead, a simpler system is used. For each carbon atom, two bonds are treated as being in the "plane of the paper" while the other two are in front of and behind that plane; such bonds are represented by thickened and dashed lines respectively.
2-Methylcyclopentanol contains a ring of carbon atoms, and atoms bonded to the ring can be either above or below the plane of the ring. This means that the methyl and alcohol groups can be on the same face of the ring (cis) or on opposite faces (trans). The two arrangements are shown in a typical fashion in Figure 2. Such diagrams not only give a lot of structural information, but are easy to draw even for an artistic incompetent.
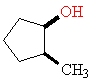 cis
cis
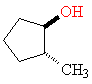 trans
trans
Figure 2. 3-D representations of 2-methylcyclohexanol. The astute observer will notice that there are one more trans isomer and one more cis isomer.
|
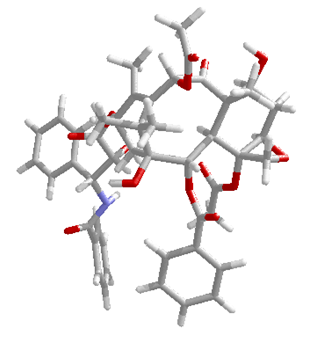
| Figure 3. "True-perspective" model of taxol. Only bonds are shown explicitly; atoms are represented by vertices. |
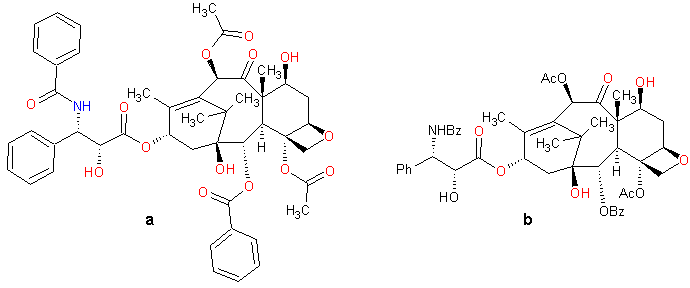
Figure 4. Schematic structure of taxol.
The essential feature of a chemical drawing is the ability to represent three-dimensional structure in a few seconds, without extensive artistic training. Most chemists think in these pictures–and their thought is constrained by them. But such constraints are no more onerous than the constraints imposed on the poet by language, on the painter by pigments and brushes, or on the sculptor by a piece of stone and a chisel. As long as we can think of things that don't exist–or things that might exist someday–we cannot say that we have reached the limits of representation.
Yet a chemical drawing is something more. Like a cartoon, it can represent much content by a few well-chosen strokes of a pencil; like the beloved icons of Eastern Christianity, it is a window to the unseen. It depicts, in stylized schematic, the essential structure of a chemical compound, allowing a creative chemist to see other structures: structures from which a compound might be built; structures into which it might be transformed.
Chemistry has a claim to be one of the visual arts, not only because of its ability to create structures of great beauty but because of the way its practitioners think, not in words but in pictures.