Stabilizing the unstable by bulking up
Very large substituents can stabilize structures that would otherwise be unstable, or even nonexistent: for example, disilene H2Si=SiH2 does not exist in the solid state -- it polymerizes into [SiH2]n. But the very bulky 2,4,6-trimethylphenyl ("mesityl," Mes) substituent allows crystalline Mes2Si=SiMes2 to be isolated.
"Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double Bond." West, R.; Fink, M.J.; Michl, J. Science 1981, 214, 1343-1344
Similarly, a striking difference is observed in the isomers of benzene and hexa-tert-butylbenzene. While benzene itself is the most thermodynamically stable of the 200+ isomers of C6H6, it is rendered rather unstable by exchanging its hydrogens for tert-butyl groups. Specifically, Dewar benzene is more than 300 kJ/mol less stable than benzene itself, but hexa-tert-butyl Dewar benzene is about 100 kJ/mol more stable than hexa-tert-butylbenzene.
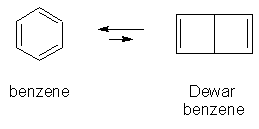
energies calculated at the HF/3-21G//HF/6-31+G** level
In this case, bulky substitutuents give thermodynamic instability to what would normally be the most stable isomer of C6H6. But very large substituents can also stabilize an unstable compound at the kinetic level, by making it less accessible for reaction -- as in the instance cited of disilene vs. tetramesityldisilene (above).