- The lowest-energy MO always has all p orbitals in phase, making it symmetric with respect to end-for-end reflection:
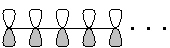
This MO has no "vertical" nodes, that is, points along the chain at which neighboring p-orbitals are out of phase. 
The next MO has a single vertical node, and one half of the MO is the "anti-mirror image" of the other half; that is, the MO is antisymmetric with respect to end-for-end reflection. An example is the π* MO of ethylene, shown above.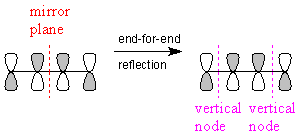
The third MO has two vertical nodes, and is symmetric. To find the position of the vertical nodes, remember that half of the p-orbitals need to reflect the other half. An example is the third MO of butadiene, shown above.- Molecular orbitals continue to add vertical nodes and alternate symmetric/antisymmetric until the highest-energy MO is reached, which has a vertical node between each atom. When combining n p orbitals, the highest-energy π MO will have n-1 vertical nodes.
Some examples of π MO diagrams are linked below. "S" is "symmetric", "A" is "antisymmetric" to end-for-end reflection (useful for thinking about disrotatory pericylic processes). Symmetries with respect to 180° rotation (useful for thinking about conrotatory processes) are the opposite.
- Ethylene (CH2)2
- Allyl (propenyl) (CH2)3
- Butadiene (CH2)4
- Pentadienyl (CH2)5
- Hexatriene (CH2)6
- Heptatrienyl (CH2)7
