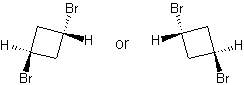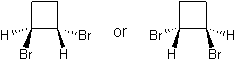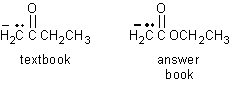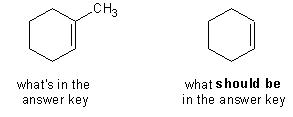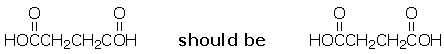Errata in Bruice, Organic Chemistry 5th Ed.
Finding an error in Bruice, or the study guide, is worth 5 points if that error is confirmed by Dr. Berger
and is not already listed on this page.
Errors in the text
Error citations are in the 2nd printing and may have already been corrected in subsequent printings.
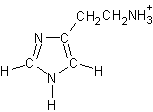 page 69, Chapter 1, problem 88. The structure of histamine is not correctly drawn. The correct structure is shown at right.
page 69, Chapter 1, problem 88. The structure of histamine is not correctly drawn. The correct structure is shown at right.- page 70, Chapter 1, problem 95. "C6H11NH3" should be C6H11NH3+.
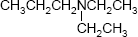 page 91, Chapter 2, problem 22e. The structure shown in the problem is incorrectly named in the Answers to Selected Problems, page A-25. The answer given is "ethylmethylpropylamine; N-ethyl-N-methylpropanamine." The correct answer is "diethylpropylamine; N,N-diethylpropanamine."
page 91, Chapter 2, problem 22e. The structure shown in the problem is incorrectly named in the Answers to Selected Problems, page A-25. The answer given is "ethylmethylpropylamine; N-ethyl-N-methylpropanamine." The correct answer is "diethylpropylamine; N,N-diethylpropanamine."- page 157, Chapter 3, problem 51. The answer in the study guide is in error as noted below; however, there appears to be typo in the text, as an entropy of 25 kilo-calories per mole per kelvin is humongous. The author probably meant to say 25 calories per mole per kelvin, which would account for the inclusion of an otherwise inexplicable factor of 10-3 in the answer key.
- page 265, Chapter 6.5, last line of the last paragraph should read
"ΔG‡alkyne > ΔG‡alkene"
- page 472, Chapter 10, problems 40 & 42. The word "alkyl" should be "aryl." Note that benzyl and allyl halides also undergo the Suzuki reaction (problem 40). This error is present in the 3rd printing as well as the 2nd.
- page 501, Chapter 11, Example 4. There is a hydrogen missing from ethylbenzene:

-
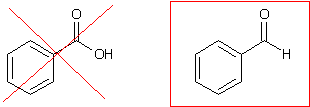
The structure of benzaldehyde at the top of page 652 is incorrect. The correct and incorrect structures are shown below.
- page 804 (Chapter 17), last paragraph. The text uses "amine" rather than "amide." The paragraph should read
The mechanisms for the reaction of LiAlH4 with unsubstituted and N,N-disubstituted amides are somewhat different... (emphasis shows the corrected word)
- page 886, Chapter 18, Mechanism for the malonic ester synthesis:
The second structure is missing an oxygen from the left-hand ethoxy group, that is, "C2H5" should be "C2H5O"
-
page 887, Chapter 18, mechanism:
In the second structure, the negatively-charged carbon should have only a single hydrogen (CH), not two hydrogens (CH2).
Errors in the study guide
Page references are to the 4th printing of the Study Guide. I include citations of errors in earlier printings, for those who are using them.
- page 97, Chapter 3, problem 51. There should be no factor of 10-3 in the entropy! See the entry under "Errors in the text," above.
- page 133, Chapter 4, problem 40, a. The correct "epoxy" name is 3,4-epoxy-4-ethyl-2-methylhexane.
- page 165, Chapter 5, problem 59a: older versions of the answer book show two chlorines and one bromine for one of the structures. This has been fixed in the newer editions.
- page 170, problem 69a: the 14C-labeled citric acid product is S, not R. Both CH2COOH groups take lower priority than the COOH group.
- page 176, Chapter 5, problem 83d: while you are asked to draw "a chiral isomer of 1,2-dibromocyclobutane," the answer key gives you structures of trans-1,3-dibromocyclobutane -- which cannot be chiral because of its symmetry.
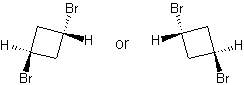
incorrect structures
|
|
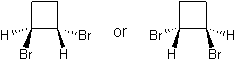
correct structures
|
- page 191, Chapter 6, problem 27e: the correct name is 1,5-cyclooctadiene, not 1,6.
- page 192, Chapter 6, problem 34e, the answer should be
1-methyl-1,3,5-cycloheptatriene
- page 218, Chapter 7, problem 47, #10. The answer key has mistakenly inserted an oxygen atom between the carbonyl group and the ethyl group. This does not change the essentials of the answer.
- page 282, Chapter 9, problem 12, from p. 406 in the text: on p. 282 of the Study Guide, the answers for parts a-2 and a-3 are switched.
- page 289, Chapter 9, problem 32b. Somehow or other, the answer-key guys started with six carbons and ended with seven. The correct answer is cyclohexene, not 1-methylcyclohexene.
- page 324, Chapter 10, problem 51c: In older printings, the second structure in the answer should be cyclohexanol, not cyclohexene. This has been fixed in the newer editions.
- page 366, Chapter 12, problem 7, 35Br and 37Br should be 79Br and 81Br. This does not affect the result of the problem, only the 50:50 ratio between the isotopes matters!
- page 489, Chapter 16, problem 64d has an incorrect structure:
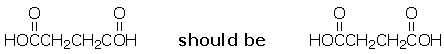
- page 501, Chapter 16, problem 87, from p. 787 in the text: the final structure in the synthesis of saccharin is missing one of the aromatic ring bonds. You should be able to figure out which one...
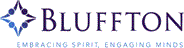
 page 69, Chapter 1, problem 88. The structure of histamine is not correctly drawn. The correct structure is shown at right.
page 69, Chapter 1, problem 88. The structure of histamine is not correctly drawn. The correct structure is shown at right.