Detailed information is given throughout Bruice, and collected in Appendix II. Qualitative acid-base behavior can be summarized thus:
You need to know what is more acidic than what else. In rough order of decreasing Brønsted acidity:
| hydrohalic acids, mineral acids (except H2CO3, HCO3- and HPO42-) | pKa -3 to ~-10 |
| protonated alcohols (ROH2+ and H3O+) | pKa ~ -2 |
| HF (hydrofluoric acid) | pKa ~ 3 |
| carboxylic acids RCOOH and H2CO3 | pKa ~ 4-5 |
| phenols (aromatic alcohols, OH on a benzene ring), thiols RSH, bicarbonate HCO3-, monohydrogen phosphate HPO42-, and ammonium cations R3NH+, R2NH2+, RNH3+, NH4+ | pKa ~ 8-10 |
| aliphatic alcohols ROH (including water) | pKa ~ 15-18 |
| saturated hydrogens α to carbonyl groups, RCOCR'2-H aldehydes and ketones are more acidic than carboxylic acid derivatives | pKa ~ 17-25 |
| terminal alkynes ( |
pKa ~ 25 |
| amines R2NH, RNH2, NH3. The hydroxide anion OH- is also around here as a Brønsted acid. | pKa ~ 36-38 |
Vinylic hydrogens R2C=CR-H are slightly more acidic than alkyl hydrogens R3C-H, but in practice neither can be removed by most bases. Their conjugate bases will deprotonate OH- to give the oxide dianion O2-. Hydride bases (LiH, NaH, KH) also fall into this category, and have pKa ~ 42. |
pKa ~ 40-60 |
 Once you know the order of acidity, you know the strength of the conjugate bases (conjugate bases of strong acids are weak; conjugate bases of weak acids are strong).
Once you know the order of acidity, you know the strength of the conjugate bases (conjugate bases of strong acids are weak; conjugate bases of weak acids are strong).
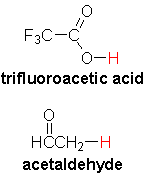
Remember that there are other factors, such as conjugation or electronegative substituents, that can make an acid much stronger. For example, trifluoroacetic acid is as strong as the mineral acids, with a pKa ~ 0.5. Acetaldehyde has a pKa of 17, in the alcohol range!
This class of organic acids--hydrogens α to carbonyl groups--is so important that it rates a whole chapter in Bruice, Chapter 18.