R. Robinson, Nature 1947, 159, 400-1
copyright © 1947 Macmillan Magazines Ltd.
Structures of Ethylene Oxide and cyclopropane
THE recent communication of Walsh1 includes several statements which are open to question. The suggestion that the reactions of ethylene oxide are at present considered to be due to instability caused by ring-strain is not acceptable without qualification and explanation. Nobody, one hopes, still retains any purely mechanical idea of strain such as one finds in the springs of models. Nowadays reactions are universally attributed to the functions of electrons and nuclei, and an interpretation of the special reactivity of such a series as acetylene, ethylene, cyclopropane and cyclopentane can surely be found by the application of quantum mechanical principles to the usual formulæ. These represent nothing more than the mode of linking of the atoms in the molecule, combined with an indication of the degree of symmetry of the statistical distribution of the electrons. Thus we may be confident that the ring-binding in cyclopropane has the symmetry of an equilateral triangle, that in ethylene oxide of an isosceles triangle, and that of propylene oxide of a scalene triangle. The usual formula for cyclopropane is therefore as satisfactory a symbol as can be devised.
The statement that trimethylene oxide is less stable than ethylene oxide is surely incorrect. Is this a case of the old confusion between stability (or reactivity) and ease of formation?
Again, the reactions of ethylene oxide are not to be compared with those of ethylene as stated by Walsh; they are, however, very similar to those of acetaldehyde. Under ordinary conditions of temperature and pressure, the olefines are anionoid in the first phases of reactions, whereas the aldehydes and ketones, and also the olefine oxides, are cationoid. That is, a reaction of ethylene is normally initiated by electron donation; a reaction of ethylene oxide by electron acceptance. The case of reaction with acids is exceptional in that a proton is probably first taken up by the non-bonded oxygen electrons, and in the resulting complex the oxygen-carbon link possesses enhanced cationoid character.
The claim that the conception of electron donation by bonding electrons is novel can be admitted only if it is limited to such new structures as those now proposed for ethylene oxide and cyclopropane. As a stage in reaction mechanism the idea can be traced back to Kekulé, whose theory of the formation of additive complexes and their redistribution can only be translated in modern terms by the use of reactive bonding electrons. If it were otherwise, saturated compounds could undergo no chemical transformations. Although it is true that a proton can often be regarded as a point of entry into the molecule, yet it is not difficult to find cases where the activity of a covalent link, carbon to carbon, should be assumed. For example, in 1916 I postulated2 the conjugation of the unsaturation of cyclopropane and cyclobutane rings with an ethenoid group, and gave as an example of conjugated addition to such a system the formation of pinene hydrochloride, which occurs with intramolecular rearrangement.
As a written symbolic expression, the formula (I) for ethylene oxide appears to be quite unsatisfactory. As pointed out by Walsh, (I) shows an analogy with the amine oxides, but the behaviour of ethylene oxide does not support the implications. There is, no doubt, a small dipole moment, but not that expected of a molecule containing → O-. The amine-oxides are easily reduced to amines; ethylene oxide is not reduced to ethylene but rather to ethanol, and that with no great ease. The very natural extension to the azoxy compounds would be incorrect. These substances cannot be formulated as (III); they have been proved to be (IV).
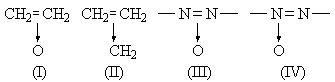
The formula (II) for cyclopropane is still less satisfactory. It does not express the symmetry of the molecule, and an attempt to achieve this by writing two other formulæ alongside would be nothing more than an admission of that degree of symmetry and in effect a reversion to the accepted structure.
What we need here is not new formulæ to set down on paper, but an explanation on quantum mechanical principles of the degree and quality of reactivity exhibited by assemblages of nuclei and electrons having the overall or average symmetry and spatial arrangement indicated by the usual structures and models. The overlapping of the orbitals would appear to supply this without the necessity for writing new constitutional formulæ.
R. ROBINSON
Dyson Perrins Laboratory,
University,
Oxford.
Feb. 10.
- Walsh, Nature, 159, 165 (1947).
- Robinson, J. Chem. Soc., 109, 1042 (1916).