I. Modes of heat transfer
- Conduction
- Heat can be conducted between two bodies which are in contact with each other; heat "flows" from one to the other.
- Materials which conduct heat well are called conductors of heat. Electrical conductors (such as metals) are good conductors of heat.
- Materials which do not conduct heat well are called insulators. Electrical insulators (for example, wood or glass) are usually good insulators of heat. Materials with low density, such as air or foamed plastic, are normally also good insulators unless they happen to be electrical conductors. To prevent heat from moving from one place to another, we usually place an insulator between.
Once a good insulator becomes hot, however, it stays that way for a long time, because it is difficult for the material to lose heat by conduction. Think of a hot ceramic pan and a hot metal pan: which cools faster?
- Convection
-
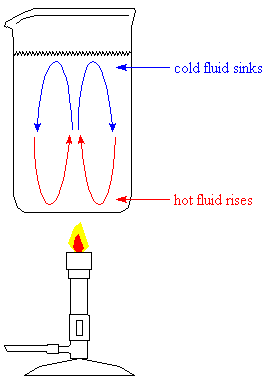 This is a different kind of heat transfer than conduction. In conduction, heat itself is moving; in convection, hot portions of a fluid move through the body of the fluid. The hot fluid mixes with the cold fluid, and heat is transferred more quickly than by conduction.
This is a different kind of heat transfer than conduction. In conduction, heat itself is moving; in convection, hot portions of a fluid move through the body of the fluid. The hot fluid mixes with the cold fluid, and heat is transferred more quickly than by conduction.
What we commonly call a "rolling boil" results from convection. Hot fluids rise through surrounding, cooler fluid because they are less dense; cooler fluids sink through warmer fluids because they are more dense. This causes circular motion of the fluid away from a source of heat. Convection in water drives ocean currents; convection in air drives weather patterns; and convection of molten rock inside the earth is thought to drive plate tectonics.
Convection leads to the counterintuitive fact that good insulators (like air) can transfer heat efficiently -- as long as the air is allowed to move freely. Trapped air, as between panes of a double window, cannot transfer heat well because it cannot mix with air of a different temperature.
- Radiation
- Radiation is the simplest means of heat transfer. Heat radiation is carried not by moving atoms (as in conduction or convection) but by electromagnetic waves. Radiation is the only way that heat can move through a vacuum, and is the reason that even a closed thermos bottle (which has a vacuum between the inner and outer parts) will eventually come to the same temperature as its surroundings.
Heat transfer is most efficient by convection, then by conduction; radiation is the least efficient and slowest means of heat transfer. Low efficiency of heat transfer means that vacuums make excellent insulation.
|