|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
The two possible geometries of 2-butene are cis-2-butene and trans-2-butene; cis indicates that substituents are arranged on the same side of the double bond, while trans indicates opposite sides. These are stereoisomers; the atoms are connected in the same way, but arranged differently in space.
For alkenes, there is a more general way of indicating cis/trans orientation: E/Z nomenclature. Z stands for the German word zusammen (together) and corresponds to cis; E stands for entgegen (opposite) and corresponds to trans. The use of these designations is discussed in your texts and will be covered in lecture; for our present purpose it is sufficient to point out that another way of naming the two diastereomers of 2-butene is as Z- or E-2-butene.
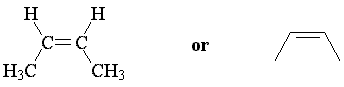 |
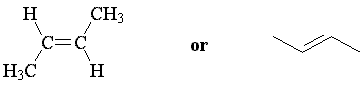 |
|||
| cis-2-butene Z-2-butene |
trans-2-butene E-2-butene |
|||
 |
 |
|||
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.