|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
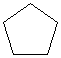
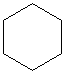
You should use the odd-colored flexible pieces in your model kit to form small (3- and 4-membered) rings, as the strain is likely to break the normal, less flexible pieces. See your kit manual for guidance.Build models of cyclopropane and cyclobutane using the flexible pieces to form the rings. Build cyclopentane and cyclohexane using the normal tetrahedral pieces.
Notice, from the models you have built with your model kit, how the rings get "floppier" as they get larger. The molecules are shown below as line drawings, in which each vertex represents a carbon atom, and hydrogens are automatically assumed to exist at unfilled carbon valences. You will see that your Molecular Visions kit works very much like a line drawing.
 |
 |
||
 |
 |
 |
 |
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.