|
|
Organic ChemistryMolecular Models: Fischer Projections |
| Return to previous page | Return to Index | Go to Fischer projection exercises |
The following pair of Fischer projections represents a rotation by 90°; click on them to see and compare the corresponding models.
The following pair of Fischer projections represents a rotation by 180° in the plane of the paper; click on them to see and compare the corresponding models.
The following pair of Fischer projections represents a rotation by 180° out of the plane of the paper; click on them to see and compare the corresponding models.
This is equivalent to rotating around a single bond.For example, if we take the Fischer projection of one of our 2-bromobutanes and perform such a rotation, we can easily see that it is S-2-bromobutane. (Remember that, in the projection on the right, the H atom is pointing away from us.)
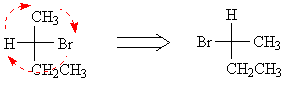
Click here to see the models corresponding with the Fischer projections.
| Return to previous page | Return to Index | Go to Fischer projection exercises |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.