|
|
Organic ChemistryMolecular Models:
|
| Return to previous page | Return to Index | Go to next page |
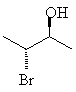
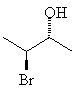
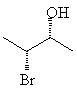
For example, 3-bromo-2-butanol, with two chiral centers, will have 22 = 4 stereoisomers. If each chiral center can be either R or S, obviously the stereoisomers will be RR, SS, RS and SR. These four are shown below; notice that each horizontal pair of isomers is a pair of enantiomers.
 |
 |
 |
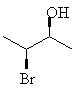 |
Assign the correct RS designation to each of the chiral centers in the molecules above, and name the molecules correctly.Remember that stereoisomers which are not mutually mirror images are called diastereomers. The top two molecules are diastereomers of the bottom ones.
| Return to previous page | Return to Index | Go to next page |

|
Dan Berger's home page |
|
Dan Berger's chemistry pages |
|
Bluffton organic chemistry pages |
Copyright © 1998, 1999, 2000, 2003, 2007 by Daniel J. Berger. This work may be copied without limit if its use is to be for non-profit educational purposes. Such copies may be by any method, present or future. The author requests only that this statement accompany all such copies. All rights to publication for profit are retained by the author.