A.D. Walsh, Nature 1947, 159, 165
copyright © 1947 Macmillan Magazines Ltd.
Structures of Ethylene Oxide and Cyclopropane
THE formula of ethylene oxide is commonly portrayed as (I), the instability of the compound being ascribed to the existence of strain tending to open the ring. This explanation can scarcely be accepted, for, if it were true, the compound (II), with less angular strain, should be more stable - which it is not. Zimakov1 has recently directed attention to this point and to the discrepancy between the structure (I) and the observed properties of ethylene oxide. He points out that the latter (for example, polymerization, addition reactions) strongly suggest a resemblance of structure to ethylene, and proposes to replace (I) by a resonance hybrid of (I), (III) and (IV).
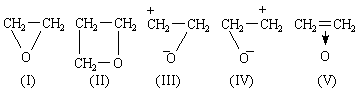
The concept of the 'co-ordinate link' type of bond is well accepted in chemistry, and is indeed necessitated by the existence of such compounds as BF3.NH3. It has hitherto been supposed that the 'donated' electrons must be initially non-bonding, but in molecular orbital theory there is nothing to warrant this limitation. In a recent paper2 we have generalized the concept to include also donation by bonding electrons and have given many illustrations of problems that then receive a simple explanation. Dewar3 has independently put forward similar ideas, and must be regarded as the first to develop the new theory. It is true that donation by lone pair electrons is more commonly observed, but this is because such electrons are usually more weakly bound than bonding electrons. It is the study of ionization potentials that provides the knowledge that enables one to say which electrons in a molecule are most likely to be donated. For donation by bonding electrons to occur, it is essential that these electrons should have especially low ionization potentials. π-Electrons in olefines fulfil this condition, though there appears to be no need strictly to limit such donation to π-electrons.
The π-electrons of ethylene lie in an orbital of ionization potential 10.45 V.4: the lone pair electrons of ammonia lie in an orbital of ionization potential 10.8 V.4. We therefore expect donation of the π-electrons of ethylene, just as donation of the lone pair electrons of ammonia occurs. Just as NH3 forms NH4+, (C=C) → H+ is expected and plays an important part in acid-catalysed olefine polymerization2. BF3.C2H4 is expected, just as BF3.NH3 is known. The formula of ethylene oxide may be portrayed as (V), and an analogy may be drawn between it and the amine oxides. (V) is closely analogous to the complex (VI) that probably plays an important part in the bromination of olefines3. The formula means that the two electrons of the original π-bond in ethylene now bind the three nuclei C, C and O. This need cause no surprise when the modern knowledge of conjugated molecules and non-localized bonds is recalled. Each carbon atom is in the trigonal state, forming three planar, hybridized sp2, σ-bonds at 120°. This is in contrast to (I), where ∠ HCH should be close to 109°. The fourth valency of each carbon atom is a pure 2pz orbital of axis at 90° to the plane of the molecule. These 2pz orbitals of the carbon atoms overlap with each other and with the 2pz atomic orbital of an oxygen atom lying alongside with the correct orientation. We therefore construct a non-localized molecular orbital, described in the LCAO approximation as
![]()
The trigonal configuration of the two carbon atoms and the existence of π-electrons account for the striking parallel between the properties of ethylene oxide and those of ethylene. The formula (V) is simpler than the cumbrous resonance description in terms of (I), (III) and (IV), though it may amount to much the same thing in ultimate thought content.
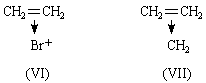
We have already stressed2 the importance of the idea of donation of π-electrons to the understanding of the reactions of the methylene molecule. We regard cyclopropane, like ethylene oxide, as having a perturbed ethylene structure (VII). Two very strong pieces of evidence for this view are that (1) substances in which a cyclopropane group is adjacent to a double bond exhibit just those spectroscopic properties which are characteristic of conjugated compounds; (2) the dipole moment of cyclopropyl chloride is 0.3 D lower than the value for isopropyl chloride5: this is easily understood on analogy with phenyl chloride, but very difficult to understand5 on the conventional formula for cyclopropane.
Further discussion of these points will be given elsewhere.
A. D. WALSH
Laboratory of Physical Chemistry,
Cambridge.
Dec. 7.